What We Do?


Calibration


Validation

Maintenance
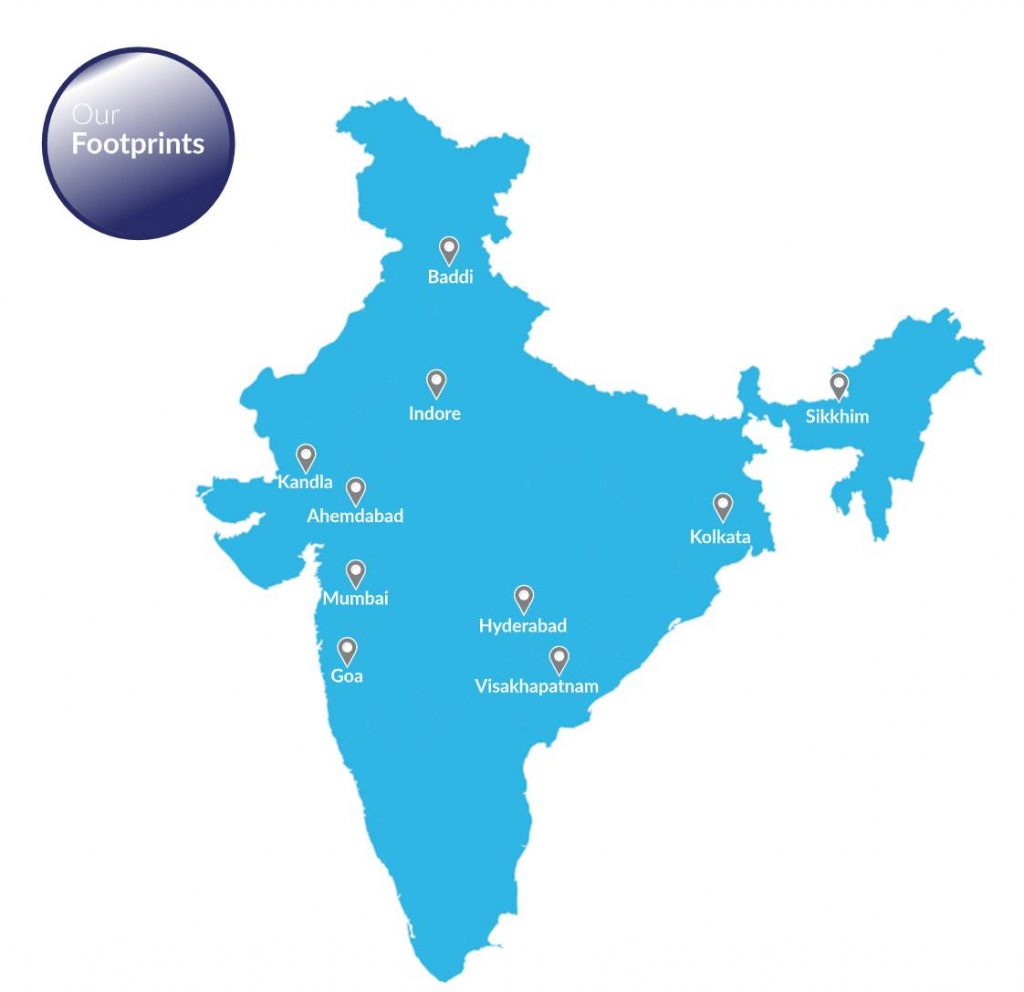
Our Footprints
- Baddi
- Indore
- Kandla
- Ahemdabad
- Mumbai
- Goa
- Hyderabad
- Visakhapatnam
- Sikkim
- Kolkata

Xpert Overview
Since its inception in 1999, Xpert Scientific Private Limited has grown from strengths to strengths to be recognized as one of the progressive manufacturers and service providers of Scientific and Clean Room Equipment. Our expertise at innovative and fast-changing technology has enabled us to provide effective solutions in stipulated time frame backed by a professional after-sales service team.
From an absolute instrument calibration service provider to the automative and pharmaceutical industries in 1999, we expanded our services with HVAC (Heat, Ventilating, and Air Conditioning) System Services in 2006.
By 2011, we were successful in setting up a fully equipped manufacturing unit along with an excellent service providing team. Today, Xpert Scientific Private Limited has forget a lucrative brand with an excellent clientele base.


